 |
Home | Contents | Orders | Customer Comments | News and Information |
Growth of
Alkaline-Earth Vanadate Garnet by Synthetic Contact Metamorphism with Molten V2O5-Na2SiO3
Acting on Dolomite
T. F. Ciszek
Geolite, P.O. Box 1453, Evergreen, CO 80437
http://www.geolite.com, info@geolite.com
________________________________________________________________________
Abstract
We have found that the garnet-structured compound Ca2NaMg2V3O12 is amenable to growth in the laboratory by synthetic contact metamorphism using a solution of V2O5 and Na2SiO3 in the approximate ratio 4.3:1 acting on dolomite marble “rock” composed of roughly equal amounts of calcium carbonate and magnesium carbonate. Cooling a 320 g crucible-charge at -2.6oC۰min-1 from a soak temperature of 1020oC yielded drusy clusters of crystals exhibiting characteristic {110} dodecahedral and {211} trapezohedral garnet faces, with individual crystals as large as 5 mm. X-ray powder diffraction confirmed a cubic structure with lattice constant = 12.427 Angstroms. The crystals had a specific gravity of 3.43 g۰cm-3, refractive index >1.8, and Mohs hardness about 4.
1. Introduction
Many silicate garnets, particularly those of the grossular (Ca3Al2Si3O12), spessartine (Mn3Al2Si3O12), and andradite (Ca3Fe2Si3O12) families, form naturally in the Earth by contact metamorphism. In this process, magmas intrude into rocks, and the reactions near the contact region sometimes result in recrystallization and garnet growth. This process is difficult to reproduce in the laboratory because of the high pressures and long time scales involved in silicate crystallization.
Recently (1998) the International Mineralogical Association added a new natural mineral Schaferite to its approved list. This vanadate garnet-structured compound Ca2NaMg2V3O12 is named after Helmut Schäfer (b. 1931) who discovered it in the volcanic Eifel Mountains of Germany [1]. Actually, Vanadate garnets with complete substitution of the pentavalent cation V5+ for Si4+ were synthesized well before the natural minerals were discovered. Bayer [2] made Ca2NaMg2V3O12 with small crystallites by solid-state reactions, to obtain materials for x-ray powder diffraction studies in 1965. Appropriate oxides and NaNO3 were heated at 700 – 750oC in oxidizing atmospheres for 50 hr. Later, Havlicek et. al. used the flux method to grow Ca2NaMg2V3O12 crystals from 1 Ca2NaMg2V3O12 : 1.5 V2O5 for EPR studies of the V4+ ion in garnet [3].
We found that Ca2NaMg2V3O12, unlike silicate garnets, is amenable to growth in the laboratory by synthetic contact metamorphism using “magma” composed of V2O5 and Na2SiO3 acting on dolomitic marble “rock” or alternatively on dolomitic limestone. Dolomite is composed of roughly equal amounts of calcium carbonate and magnesium carbonate. The relatively low maximum temperature required (1020oC) is convenient for lab scale simulations of contact metamorphism.
2. Experimental Procedure
V2O5 and Na2SiO3 powders were thoroughly mixed in the weight ratio 4.3 : 1, and 185 g were tamped in a 250 cm3 crucible (along with a small amount of Cr2O3 dopant to add color to the crystals). Three pieces of white dolomite marble weighing a total of 90 g and four pieces of crystalline quartz weighing a total of 30 g were placed on top of the powder. The quartz does not appear to enter the reaction, but was used to provide crevices and surfaces for the crystal growth. The assembly was heated gradually to 1020 oC in a resistance-heated box furnace, held for 9 hours, and then cooled at a steady rate of -2.6oC/h until the temperature reached 763oC. At this point, the saturated “magma” was poured off and the crucible was put back into the furnace for slow cooling with the power disconnected.
3. Results and Discussion
Clusters of orangey-red crystals with dodecahedral {110} and trapezohedral {211} typical garnet faces were formed in the crucible, and were evident after the pour without any additional steps to remove residual “magma” (see Fig. 1). Some flattened crystals also grew near the liquid surface. Substantial reaction with, and consumption of, the dolomite was evident. Little, if any, quartz crystal consumption took place. Individual crystals up to about 5mm in width were obtained.
3.1
X-ray Analysis
Some crystals were crushed to a powder for x-ray determination of the crystal structure. A powder pattern was obtained using Cu KaI radiation for 2Q angles between 10 and 90 degrees, and is shown in Fig. 2 as a continuous line plot of diffracted intensity vs. 2Q. Bayer’s 1965 JCPDS data [2] for Ca2NaMg2V3O12 synthesized by solid-state reaction is also shown in the figure, as round filled symbols with dotted drop lines to the 2Q axis. The positions and relative intensities [1] for the six strongest x-ray diffraction peaks of the natural mineral Schaferite are depicted in Fig. 2 as square, filled symbols with dashed drop lines to the 2Q axis. Correlation of line positions for the three sets of data is very good, confirming the identification of our grown crystals as cubic (isometric, 4/m 3 2/m, space group I a3d), typical garnet-structured Ca2NaMg2V3O12. All three data sets indicate a particularly high-intensity peak at 2Q ~ 32 degrees, corresponding to {hkl} = {420}. Both our crystals and Bayer’s display substantial peaks at the positions of the 6 strongest Schaferite peaks. But our crystals have strong peaks near 2Q positions of approximately 36.6, 62.3, 69.1, 78.2, and 83.6 degrees as well. These are present in Bayer’s data, but at lower intensities. Table I gives numeric values for the three data sets.
We determined a lattice constant for our crystals by plotting the lattice parameter for each peak as a function of the Taylor and Sinclair [4]/Nelson and Riley [5] extrapolation function (cos2Q/sinQ + cos2Q/Q)/2, and extrapolating the linear regression fit to a zero value of the extrapolation function. The value obtained is 12.427 ± 0.004 Angstroms. The reported lattice constant for Schaferite is 12.427 Angstroms, and the reported constant from Bayer’s JSPDS data is 12.455 Angstroms. Extrapolation plots for all three materials are shown in Fig. 3. It does not appear that the extrapolation function was used for the reported Schaferite or Bayer lattice constants.
3.2
Other physical properties
Three clean crystal fragments 4-5 mm in size were weighed in air, and also while suspended in distilled water, to obtain the crystals’ density, r = 3.43 g·cm-3. This is slightly less than the calculated density using the lattice constant 12.427 Angstroms, which gives a value of 3.44 g·cm-3.
Scratch tests were done to estimate Mohs-scale hardness of the crystals. The crystals were scratched by zoisite (hardness 6 ½), feldspar (hardness 6), lapis lazuli (hardness 5 – 5 ½), and rhodochrosite (hardness 4), but not by malachite (hardness 4). We conclude that the hardness is approximately 4 on the Mohs scale.
Examination of a crystal face with a conventional prism refractometer (upper refractive index limit of 1.8) did not yield any intensity demarcation edges. The refractive index is thus greater than 1.8. No sharp absorption lines were seen with a prism spectrometer.
Similar crystal growth results were obtained when powdered dolomitic limestone was substituted for dolomite marble in the growth process. Likewise, sodium bicarbonate (NaHCO3) was successfully substituted for Na2SiO3. Slow heat-up is necessary because substantial frothing of the melt occurs from evolved gases.
4. Summary
Garnet-structured Ca2NaMg2V3O12 was grown by synthetic contact metamorphism, using “magma” of V2O5 and Na2SiO3 in the approximate ratio 4.3:1 acting on dolomite marble “rock.” Drusy clusters of red-orange crystals up to 5mm in size were obtained. X-ray powder diffraction analysis confirmed the structure. The isometric lattice constant of the crystals is 12.427 Angstroms, the hardness is Mohs 4, the density is 3.43 g·cm-3, and the refractive index is >1.8. The natural form of this material is the mineral Schaferite. The V2O5/Na2SiO3/dolomite system is attractive for simulating contact metamorphic processes in the laboratory, because relatively low temperatures (1020 oC), fast growth rates, and inexpensive materials are involved.
References
[1]
John L. Jambor, Vladimir A. Kovalenker,
and Andrew C. Roberts, Am.Min.
84 (1999) 1685–1688.
[2] Gerhard Bayer, J. Am. Ceram. Soc. 48
(1965) 600.
[3] V. Havlicek, P. Novak, and M Vichr,
Phys. Stat. Sol. (b) 44 (1971) K21.
[4] A. Taylor and H.
Sinclair, Proc. Phys. Soc. (London) 57 (1945) 126.
[5] J. B. Nelson and D. P.
Riley, Proc. Phys. Soc. (London) 57 (1945) 160.
Table I. X-ray powder diffraction parameters for our crystals, the Bayer/JCPDS synthetic crystals, and natural Schaferite.
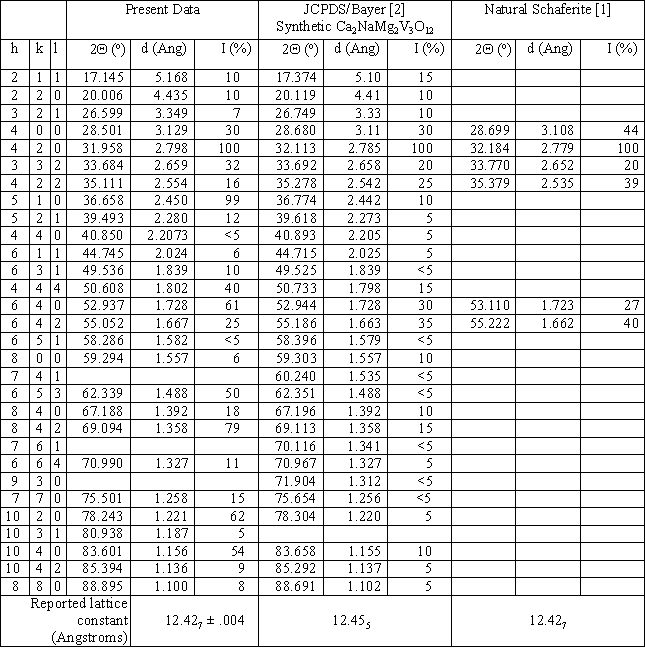

Fig. 1. Portion of a drusy crystal cluster grown from the interaction of molten V2O5 and Na2SiO3 with dolomite marble (picture width represents about 25 mm.
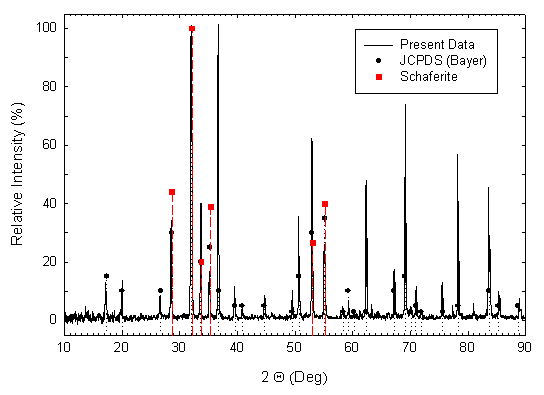
Fig. 2.
CuKaI
x-ray diffraction pattern for powder crushed from some of the crystals shown in
Fig. 1 (continuous line). The
filled-circle symbols and dotted vertical lines represent relative intensities
and 2Q
line positions for JCPDS entry 18-1204 from the data of Bayer [2] for
solid-state synthesized Ca2NaMg2V3O12.
The filled-square symbols and dashed vertical lines represent relative
intensities and 2Q
line positions for natural Schaferite [1].
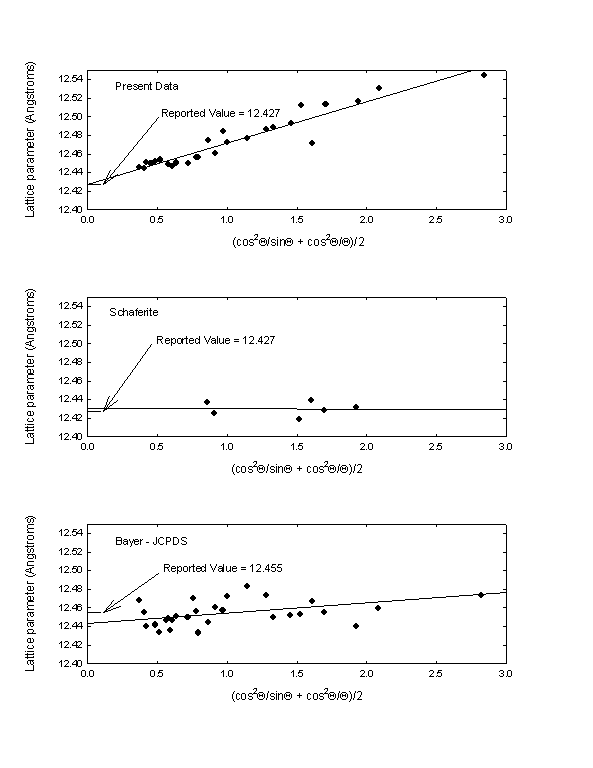
Fig. 3. Taylor and Sinclair/Nelson and Riley lattice parameter extrapolation function plots for the present data (top), natural Schaferite (middle), and the Bayer JCPDS material grown by solid-state reaction (bottom). The reported values for the lattice constant of Ca2NaMg2V3O12 from each data set are also indicated.